Intragenic viral silencer element regulates HTLV-1 latency via RUNX complex recruitment
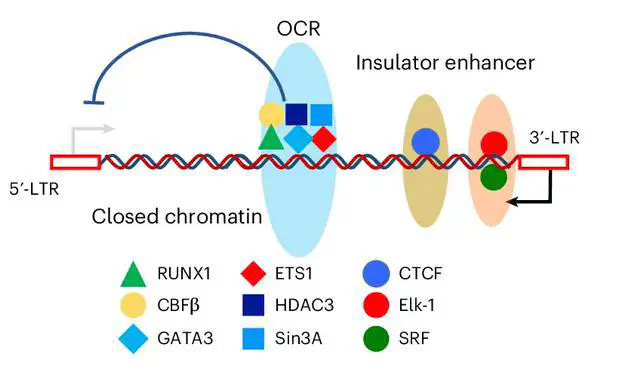
An intragenic viral silencer within the HTLV‑1 provirus recruits the host RUNX complex to dampen sense‑strand transcription and promote latency. Disrupting this element boosts HTLV‑1 expression and immunogenicity; inserting it into HIV‑1 suppresses production—revealing a portable, cis‑acting latency switch.
Abstract (concise summary)
The study maps an open chromatin region inside the HTLV‑1 provirus that functions as a silencer of the 5′‑LTR. The element binds RUNX1/CBFβ, limits transcriptional bursts and antigen expression, and thereby supports viral persistence. CRISPR mutagenesis and reporter assays show that removing the element elevates viral transcripts and replication, while heterologous insertion into HIV‑1 diminishes virion output. These results uncover a host‑factor‑tethered intragenic latency module with therapeutic implications for retroviral control.
Key takeaways
- Mechanism: RUNX complex binding to an intragenic silencer represses HTLV‑1 sense transcription and stabilizes latency.
- Portability: The element is sufficient to suppress HIV‑1 production when transplanted into the HIV‑1 genome.
- Methods: ATAC‑seq, luciferase reporters, CRISPR editing, qPCR/ddPCR, ChIP‑PCR, flow cytometry and patient PBMC analyses.
- Why it matters: Identifies a discrete, druggable node for latency control that could inform antivirals or vaccine‑adjuvant strategies.
Suggested citation
Sugata K, Rahman A, Niimura K, et al. Intragenic viral silencer element regulates HTLV‑1 latency via RUNX complex recruitment. Nat Microbiol. 2025;10(6):1447–1462. doi:10.1038/s41564-025-02006-7.